KATE KELLAND
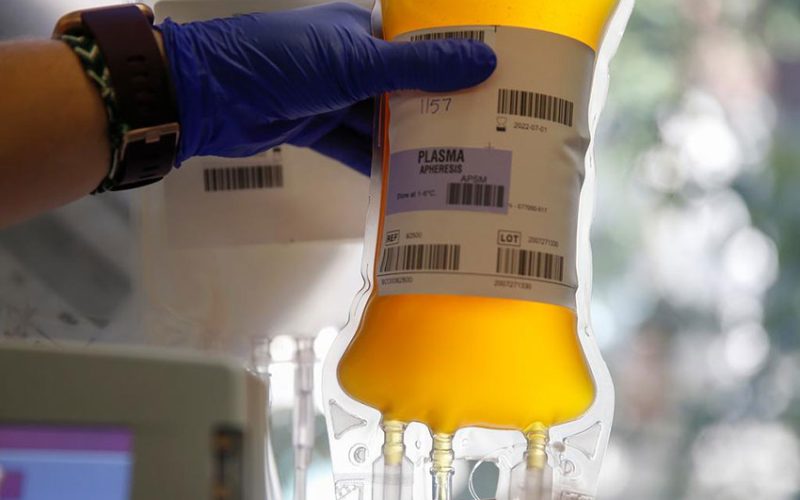
AN international trial testing convalescent blood plasma in cases of moderate and severe COVID-19 has halted enrolment of severely ill patients requiring intensive care after finding the plasma was of no benefit, trial investigators have said
The decision by the REMAP-CAP trial leaders came after an initial analysis of more than 900 severely ill trial participants in intensive care showed that treatment with the product – an antibody-rich plasma taken from people who have recovered from the pandemic disease – did not improve outcomes.
“There was no evidence of harm associated with the administration of convalescent plasma” (and) the trial is continuing to recruit hospitalised COVID-19 patients who are moderately ill but not in intensive care, scientists leading the trial said in a statement.
“It is biologically plausible that patients who are not producing antibodies at the time of convalescent plasma therapy and those patients with excess virus may benefit more than others. Our additional analyses will explore this,” said Manu Shankar-Hari, a clinician and professor of critical care medicine at Britain’s Guy’s and St Thomas’ hospital, who is co-leading the trial.
He added that the initial analysis did not assess plasma’s effects in hospitalised patients with less severe disease. This “remains a very important question” and would continue to be explored in the ongoing trial, he said.
The underlying hypothesis for using convalescent plasma as a potential treatment for COVID-19 patients is that the antibodies it contains could neutralize the virus, stopping it from replicating and halting tissue damage.
Smaller studies of convalescent plasma in COVID-19 patients in India and in Argentina have also found no apparent benefit for those critically or severely ill with the disease .
The analysis leading to the REMAP-CAP pause in enrolment of critically ill patients showed there was a very low probability – 2.2% – that it reduced death rates or decreased the number of days patients needed intensive care.
“Why convalescent plasma does not seem to improve outcome in severely ill COVID-19 patients admitted to the ICU is not yet known. However, it may be because the lung damage is too advanced for convalescent plasma to make a difference,” said Alexis Turgeon, a critical care doctor and professor at Université Laval in Canada, who is also working on the trial.
But scientists and doctors called for convalescent plasma trials to continue and for recovered COVID-19 patients to continue to donate their blood in the hope it might help others.
“Antibodies work by stopping the virus, not by treating the symptoms,” said Gail Miflin, a medical expert in the blood and transplant unit of the UK National Health Service. “The emerging evidence from international studies is that use before intensive care may prove to be more effective.”
REMAP-CAP is an international clinical trial exploring a range of potential treatments for COVID-19. It has already recruited 4,100 COVID-19 patients for convalescent plasma trials at more than 290 clinical sites across Europe, the Americas, Asia, Africa and Australasia.
Separate findings from REMAP-CAP showed last week that treating critically ill COVID-19 patients with Roche’s Actemra or Sanofi’s Kevzara arthritis drugs significantly improved survival rates and reduced the amount of time patients need intensive care.